M-Foto/Shutterstock
Since the early days of the pandemic, attention has focused on producing a vaccine for COVID-19. With one, it’s hoped it will be able to suppress the virus without relying purely on economically challenging control measures. Without one, the world will probably have to live with COVID-19 as an endemic disease. It’s unlikely the coronavirus will naturally burn itself out.
With so much at stake, it’s not surprising that COVID-19 vaccines have become both a public and political obsession. The good news is that making one is possible: the virus has the right characteristics to be fended off with a vaccine, and the economic incentive exists to get one (or indeed several) developed.
But we need to be patient. Creating a new medicine requires a large amount of thought and scrutiny to make sure what’s produced is safe and effective. Researchers must be careful not to allow the pressure and allure of creating a vaccine quickly to undermine the integrity of their work. The upshot may be that we don’t have a highly effective vaccine against COVID-19 for some time.
Here, authors from across The Conversation outline what we know so far. Drawing upon their expertise, they explain how a COVID-19 vaccine will work, the progress a leading vaccine (developed by the University of Oxford with AstraZeneca) is making, and what challenges there will be to manufacturing and rolling a vaccine out when ready.
How will vaccines work for COVID-19?
How the spike protein is produced
The benefits of different designs
Why boosters may be needed
What determines how we respond to vaccines
Why vaccines provide strong immunity
How to use a vaccine when it’s available
How is the Oxford vaccine being developed, tested and approved?
The many steps of vaccine development
The results of phase 1 and phase 2 trials
How the phase 3 trial will work
Why testing was paused – and why we shouldn’t be alarmed
Why vaccine makers need to be more open
Why we need to know what’s in placebos
How will the vaccine be made and rolled out?
How to prepare enough vaccines for the whole world
How tobacco could play a role in producing a vaccine
Why vaccines need to be kept cold
Will rich countries buy up the supply when vaccines are available?
How to stop rich countries from depriving poorer ones
Who should get a vaccine first?
How do you counter resistance and scepticism?
Vaccine hesitancy is nothing new
Are anti-vaxxers that big a problem?
How the far right is exploiting the pandemic
How to build trust in vaccines
How will vaccines work for COVID-19?
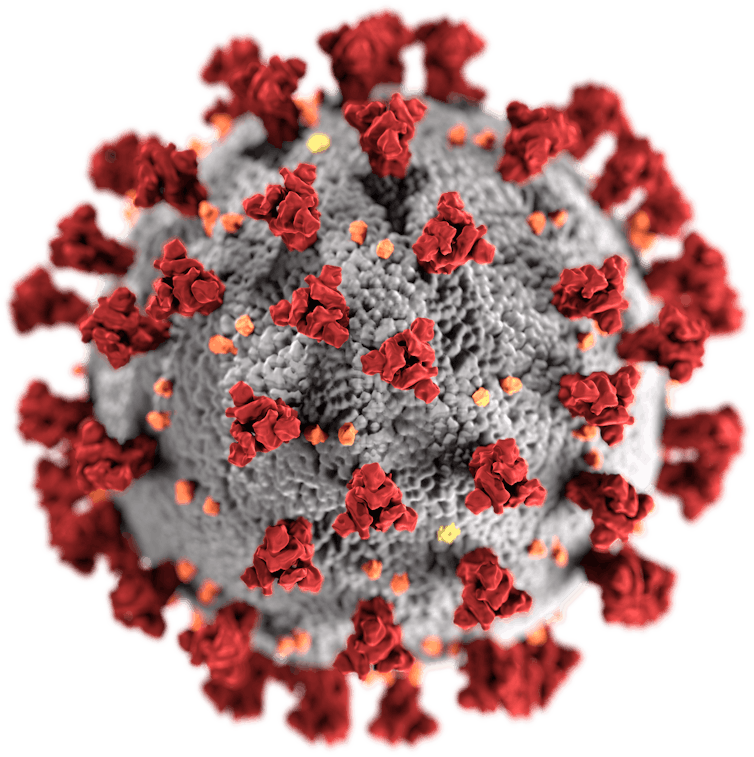
Producing the spike protein
Although the way the body interacts with SARS-CoV-2 isn’t fully understood, there’s one particular part of the virus that’s thought to trigger an immune response – the spike protein, which sticks up on the virus’s surface. Therefore, the two leading COVID-19 vaccines both focus on getting the body to produce these key spike proteins, to train the immune system to recognise them and destroy any viral particles that exhibit them in the future.
US Centers for Disease Control and Prevention/Wikimedia Commons
The pros and cons of different designs
The leading vaccines both work by delivering a piece of the coronavirus’s genetic material into cells, which instructs the cell to make copies of the spike protein. As Suresh Mahalingam and Adam Taylor explain, one (Moderna’s) makes the delivery using a molecule called messenger RNA, the other (AstraZeneca’s) using a harmless adenovirus. These cutting-edge vaccine designs have their pros and cons, as do traditional methods.
Boosters may be needed
The strongest immune responses, says Sarah Pitt, come from vaccines that contain a live version of what they’re trying to protect against. Because there’s so much we don’t know about SARS-CoV-2, putting a live version of the virus into a vaccine can be risky. Safer methods – such as getting the body to make just the virus’s spike proteins, or delivering a dead version of the virus – will lead to a weaker response that fades over time. But boosters can top this up.
What governs how we respond to vaccines?
A vaccine’s design isn’t the only factor that determines how strong our immune response is. As Menno van Zelm and Paul Gill show, there are four other variables that make each person’s response to a vaccine unique: their age, their genes, lifestyle factors and what previous infections they have been exposed to. It may be that not everyone gets long-lasting immunity from a vaccine.
Why vaccines provide strong immunity
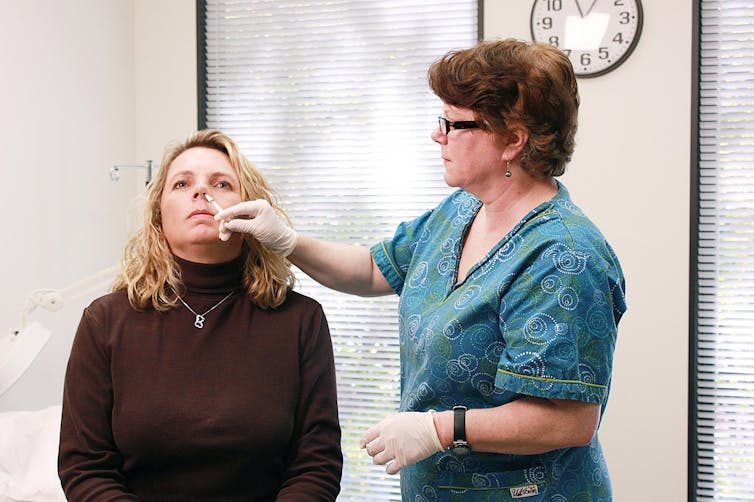
If well-designed, a vaccine can provide better immunity than natural infection, says Maitreyi Shivkumar. This is because vaccines can focus the immune system on targeting recognisable parts of the pathogen (for example the spike protein), can kickstart a stronger response using ingredients called adjuvants, and can be delivered to key parts of the body where an immune response is needed most. For COVID-19, this could be the nose.
Douglas Jordan, MA/CDC
How to use a vaccine when it’s available
Scientists think between 50% and 70% of people need to be resistant to the coronavirus to stop it spreading. Using a vaccine to rapidly make that many people immune might be difficult, says Adam Kleczkowski. Vaccines are rarely 100% effective, and hesitancy and potential side effects may make a quick, mass roll-out unrealistic. A better strategy might be to target people most at risk together with those likely to infect many others.
How is the Oxford vaccine being developed, tested and approved?
The many steps of vaccine development
Vaccine development is quicker now than it ever has been, explain Samantha Vanderslott, Andrew Pollard and Tonia Thomas. Researchers can use knowledge from previous vaccines, and in an outbreak more resources are made available. Nevertheless, it’s still a lengthy process, involving research on the virus, testing in animals and clinical trials in humans. Once approved, millions of doses then need to be produced.
Phase 1 and phase 2 trials are successful
After showing promise in animals, the University of Oxford’s vaccine moved onto human testing – known as clinical trials, which are split into three phases. Here, Rebecca Ashfield outlines the joint phase 1 and 2 trial that the vaccine passed through to check that it was safe and elicited an immune response, and explains how the vaccine actually uses a separate virus – a chimpanzee adenovirus – to deliver its content into cells.
How the phase 3 trial works
Earlier trial phases showed that the vaccine stimulated the immune system, as expected. But the million-dollar question is whether this actually protects against COVID-19. Finding out means giving the vaccine to thousands of people who might be exposed to the coronavirus and seeing whether they get sick. As Ashfield and Pedro Folegatti show, this requires running vaccination programmes in countries across the world.
Testing was paused – and that’s OK
In September, the phase 3 trial of the Oxford vaccine was paused after a patient fell ill with a possible adverse reaction. Understandably this caused dismay, but it shouldn’t have, says Simon Kolstoe. Pauses like this are common, as independent moderators are needed to assess exactly what has happened. Often illnesses in trials are unrelated to what’s being tested. But even if they are, that’s exactly what we want these tests to show.
DonyaHHI/Shutterstock
But vaccine makers need to be more open
AstraZeneca didn’t publicly reveal what caused the pause but did share this information with investors. This, says Duncan Matthews, was an example of an attempt to apply old methods of operating to a new situation.
Why we need to know what’s in placebos
A key part of clinical trials are placebos – alternative or inactive treatments that are given to participants for comparison. But a key problem, Jeremy Howick explains, is that some vaccine trials don’t reveal what their placebos contain. Without knowing what benchmark is being used, it’s then difficult for outsiders to understand the relative effect (and side effects) the vaccine has.
How will the vaccine be made and rolled out?
Preparing enough for the whole world
Universal demand for a COVID-19 vaccine means production bottlenecks are a risk. For the Oxford vaccine, production involves growing key components in human embryonic kidney cells, before creating the actual vaccine and then purifying and then concentrating it. Running this process at industrial scale, say Qasim Rafiq and Martina Micheletti, is one of the biggest challenges AstraZeneca faces.

RGtimeline/Shutterstock
Tobacco – an unexpected ally?
Vaccines contain organic products, which traditionally have been grown using cell cultures in containers called bioreactors. Recently plants have been adapted to function as bioreactors too, which could help production be massively increased. Tobacco may be especially useful: it grows quickly, is farmed all over the world, is leafy and easily modifiable. The tech hasn’t been approved for mass producing medicines – but demand may change that.
Keeping vaccines cool will be crucial
Because COVID-19 vaccines will contain biological material, they’ll need to be kept cold right up until they’re delivered, explains Anna Nagurney. Fail to keep them cool and they’ll become ineffective. Refrigeration will therefore be a major challenge in any roll-out campaign; an estimated 25% of vaccines are spoiled by the time they reach their destination. A potential solution could be to encase their heat-sensitive parts in silica.

Tony Karumba/AFP via Getty Images
‘Vaccine nationalism’ threatens universal access
Some governments are signing agreements with manufacturers to supply them with vaccines ahead of other countries. Poorer nations risk being left empty handed – putting people at risk and preventing any attempt to coordinate suppressing the coronavirus worldwide. It’s also unclear how access is being priced in these deals.
How to counter vaccine nationalism
India can play a key role in avoiding this “richest-takes-all” scenario, says Rory Horner. It’s traditionally been a major supplier of medicines to the global south, and has the capacity to create more vaccines for COVID-19 than any other country in the world. India’s Serum Institute has signed up to make 400 million doses of the Oxford vaccine this year, but with a population of 1.35 billion, how many will go abroad isn’t yet clear.
Shutterstock/ManoejPaateel
Who will get the coronavirus vaccine first?
We need to plan now, say Laurence Roope and Philip Clarke. Governments have big decisions to make. The pandemic is akin to a war situation, so there’s an argument these vital goods should be rationed and banned from private sale. Authorities also need to decide who should be prioritised: those most vulnerable, people most likely to spread the virus, or those who can kickstart the economy by returning to work.
How do you counter resistance and scepticism?
Public resistance is a sizeable problem – but nothing new
Surveys show that one in four New Zealanders remain hesitant about a coronavirus vaccine, while one in six British people would refuse one. But vaccine hesitancy has been around for a long time, writes Sally Frampton. And Steven King argues the past – such as when smallpox vaccines were resisted – may provide some solutions to this problem.
Are anti-vaxxers a problem?
Not all hesitancy is the same, says Annamaria Carusi. As well as the hardcore anti-vaxxers, plenty may resist COVID-19 vaccines on safety or animal welfare grounds. Indeed, while anti-vaxxers attract a lot of attention, their influence on vaccination rates is often overstated, argues Samantha Vanderslott. In fact, desire for a vaccine is so widespread and strong that anti-vaxxer positions may be harder to defend right now.
The far right is exploiting the pandemic
A recent report from the United Nations Security Council warned that extreme right-wing groups in the US are using the pandemic to “radicalise, recruit, and inspire plots and attacks”. Blyth Crawford gives a run-down of the major groups at work in America – what their aims are, the methods they’re using to reach people, and the key pieces of misinformation that they’re peddling.
How to build trust in vaccines
The usual strategy is to double down on positive messaging. But a better strategy, Mark Honigsbaum argues, would be to acknowledge that there’s a lot we don’t know about how some vaccines work, but that the benefits of taking vaccines far outweigh the risks. A further step could be to make sure that manufacturers are liable should vaccine recipients suffer negative effects. Often manufacturers are exempt.
Looking ahead
The future is full of possibility. COVID-19, Sars, Mers and the common cold are all caused by coronaviruses, and scientists are considering whether it’s possible to create a vaccine that could offer protection against them all – and perhaps even against an as yet unknown coronavirus we’re yet to encounter. Admittedly, having a vaccine that can do this seems unlikely in the near future.
We shouldn’t get ahead of ourselves, though, says Sarah Pitt. No vaccine has yet completed its safety trials, and we can’t yet be sure that any vaccine will permanently prevent people from catching COVID-19. We need to prepare ourselves for the very real possibility that a COVID-19 vaccine only reduces the severity of symptoms or provides temporary protection.
![]()