One of the goals of palaeontology is to bring the past to life. In recent years, some spectacular discoveries have revealed unprecedented glimpses into fossilised animals and their lives.
We know some dinosaurs cared for their young, that the earliest bees were pollinators, and what some ancient birds and crickets sounded like. But one question has remained elusive for decades: what colours were ancient beasts? For years, palaeontologists were unable to answer this, as fossils are rarely preserved with pigments or colour patterns.
In recent years, breakthroughs have helped unearth clues to the pigments found in animals’ skin, hair and feathers. But melanin pigments have also been found inside some animals, prompting the question of what their functions are inside the body.
The answer, it turns out, has changed over time. In a new study, we discovered changes in the functions of melanin over millions of years, including why it made its way into the hair of mammals and bird feathers.
Fossilised melanin
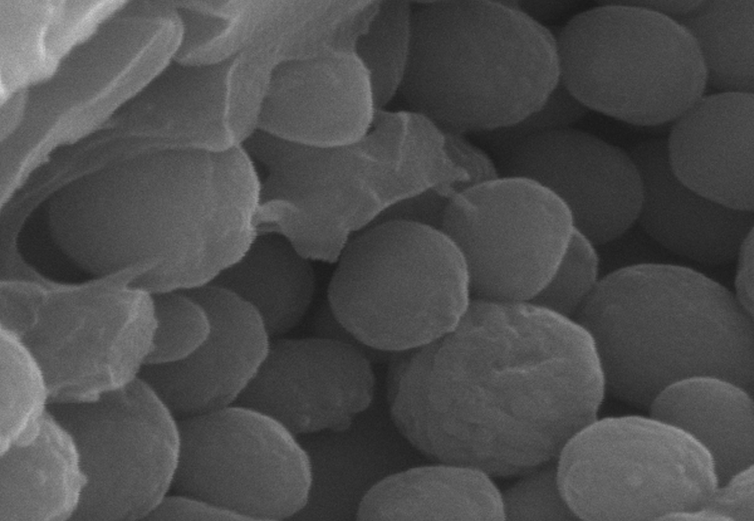
In the early 1980s, palaeontologists studying 47-million-year-old fossils identified microscopic sausage-shaped structures that looked uncannily like the bacteria found on decaying animal carcasses today. For decades, when similar structures were found in vertebrate fossils, they were interpreted as the fossil remains of decayed bacteria, preserved on the carcasses they were helping to decay.
Little did we know how wrong we were. In 2008, palaeontologist Jakob Vinther recognised the fossil structures were remarkably similar to melanosomes – granules of the pigment melanin produced in our cells that impart colour to tissues such as skin, feathers and hair. This discovery opened the floodgates for studies of fossil melanin, yielding dramatic reconstructions of the plumage colours of feathered dinosaurs, ancient birds, early fishlike vertebrates and even a fossil mouse.
Read more:
Fossils help to reveal the true colours of extinct mammals for the first time
Over the past 13 years, studies of coloration gave insights into ancient animal behaviour and feather evolution. In tandem, various research groups probed the chemistry and structure of the fossil melanosomes in more detail. Additionally, with experiments simulating the way fossils form, we’ve come to better understand the fossil record of melanin and melanosomes.
Maria McNamara, Author provided
Under the skin
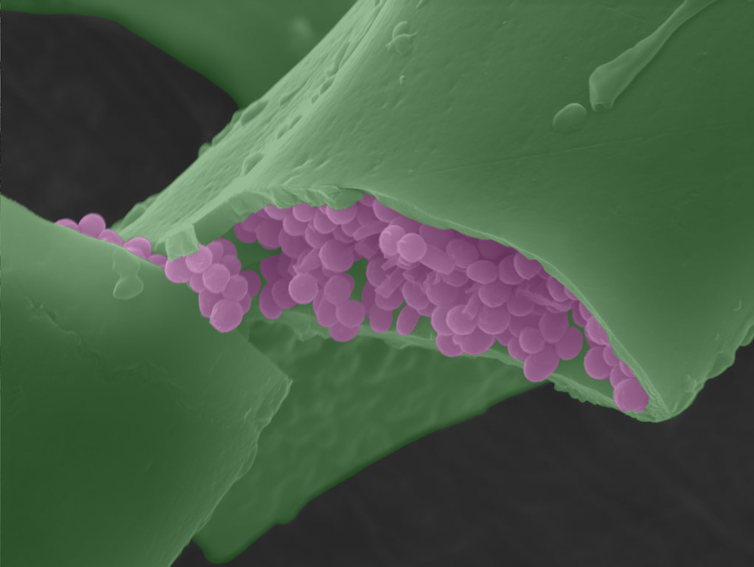
Almost all studies assumed fossil melanosomes came from tissues on the outside – skin, feathers or hair. This was overturned by our discovery in 2018 that modern frogs contain melanin inside their bodies – in the lungs, spleen and especially the liver. Some species even contain more of this “internal” melanin than in the skin. [Our study in 2019] [https://www.pnas.org/content/116/36/17880] showed internal melanins also occur in reptiles, birds and mammals.
This could have spelled the end for colour reconstruction, but luckily melanosomes from the skin are a different size and shape to internal ones. We can single out melanosomes from the skin and feathers and use these to study colour and behaviour.
What melanosomes were doing, however, in internal organs remained a mystery. Studies have shown hints of possible functions, like regulating metals in the body and acting as an antioxidant.
In a new study we looked at fossils and modern animals to model the evolution of melanin and its functions. Our study revealed a major transition in melanin evolution coincident with the evolution of warm-blooded lifestyles.
Warm blooded
In cold-blooded vertebrates, such as reptiles and amphibians, most melanin occurs in internal organs. In warm-blooded birds and mammals, most melanin occurs in hair and feathers. The reasons for this shift became clear once we considered the effects of melanin on the body.
Melanin provides lots of advantages, like colour production, metal regulation, antioxidant function, tissue strengthening and protection from sunlight. But producing and storing melanin generates toxins that can damage tissues and DNA.
When birds and mammals evolved feathers and hair, melanin storage was relocated to these dead tissues. This protected the body from damage but still allowed melanin to fulfil its key roles in protection from harmful ultraviolet (UV) rays and in metal regulation.
Why, then, don’t amphibians and reptiles store melanin in their skin? Because amphibians and reptiles depend on melanin-rich tissue structures in their liver and spleen for their immunity.
Birds and mammals evolved more sophisticated immune systems that don’t need melanin. The evolution of feathers and hair provided birds also had knock-on effects on coloration, which is still dominated by melanin in mammals and many birds.
Maria McNamara, Author provided
We also found evidence for an intriguing shift in the type of melanin used between cold-blooded and warm-blooded species. Birds and mammals contain significantly more of a sulphur-rich variant of melanin than amphibians and reptiles. This almost certainly reflects another evolutionary trade-off, as production and storage generate more toxins than the low-sulphur version. Moving sulphur-rich melanin to feathers and hair allows the production of reddish-brown and orange colours in a “safe space”, expanding the range of possible colours beyond the blacks and greys of the low-sulphur variety.
The evolution of birds and mammals has been shaped behind the scenes by intimate links between melanin and our immune systems, how we regulate our internal environment, protect against UV and generate colour.
What next?
We showed how colour patterns in fossils can be used to map the evolution of melanin genes over the last 500 million years. Many questions remain unanswered, like when birds and mammals lost most of their internal melanin.
While studying the colour of specific extinct animals will remain useful for understanding the behaviour and ecology of ancient animals, many of the most exciting questions about fossil melanin now relate to changes over millions of years. To understand pivotal events in vertebrate evolution, we need to take a closer look at melanin and its cryptic shaping of our bodies through deep time.
![]()
Maria McNamara receives funding from the European Research Council and Science Foundation Ireland. She works for University College Cork (UCC), Ireland.
Tiffany Slater is affiliated with University College Cork (UCC), Ireland.
Valentina Rossi is currently affiliated with the Museo di Scienze Naturali dell'Alto Adige, Bolzano, Italy.











